

Electron configuration notationĮlectron configurations can be written in a shorthand notation using the noble gas configuration. Similarly, in the case of copper, having a completely filled d subshell is more stable than having a partially filled d subshell. In the case of chromium, having five half-filled d orbitals is more stable than having four partially filled and one completely filled d orbitals.

These exceptions occur because half-filled or completely filled subshells are more stable than partially-filled subshells. Another example is the copper (Cu) atom, which has an electron configuration of 3d10 4s1 instead of the expected 3d9 4s2. One of the most notable is the chromium (Cr) atom, which has an electron configuration of 3d5 4s1 instead of the expected 3d4 4s2. While the Aufbau principle is generally followed, there are a few exceptions to this rule. Exceptions to the electron configuration rules On the other hand, elements with partially filled subshells, such as copper and silver, are more reactive and have high electrical conductivity. Elements with completely filled subshells, such as helium and neon, are non-reactive and have low electrical conductivity. The valence electrons determine the type of chemical bonds that an element can form and its reactivity.Įlectron configuration also affects the magnetic and electrical properties of elements. This is because they have the same number of valence electrons, which are the outermost electrons involved in chemical bonding. It determines the number and arrangement of electrons in the atom, which affects how the atom interacts with other atoms and molecules.įor example, elements with similar electron configurations tend to have similar chemical properties. The electron configuration of an element is important in determining its chemical and physical properties. The first 18 elements have simple electron configurations, while the electron configurations of heavier elements become more complex due to the additional sublevels and orbitals. The electron configuration of an element is determined by the number of electrons it has, which is equal to its atomic number. This indicates that there are two electrons in the first energy level (1s), two electrons in the second energy level (2s), and two electrons in the second energy level's p sublevel (2p). For example, the electron configuration of carbon (atomic number 6) can be written as 1s2 2s2 2p2. It can be written using a shorthand notation that indicates the energy level, sublevel, and number of electrons in each sublevel. The electron configuration of an element refers to the arrangement of electrons in its atoms. Electrons fill the s subshells before filling the p subshells, which in turn are filled before the d and f subshells. The order of filling of the subshells is as follows: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, and 7p. In other words, electrons fill up the shells and subshells in a specific order, starting from the lowest energy state. It states that electrons will occupy the lowest energy level available to them before moving to higher energy levels.
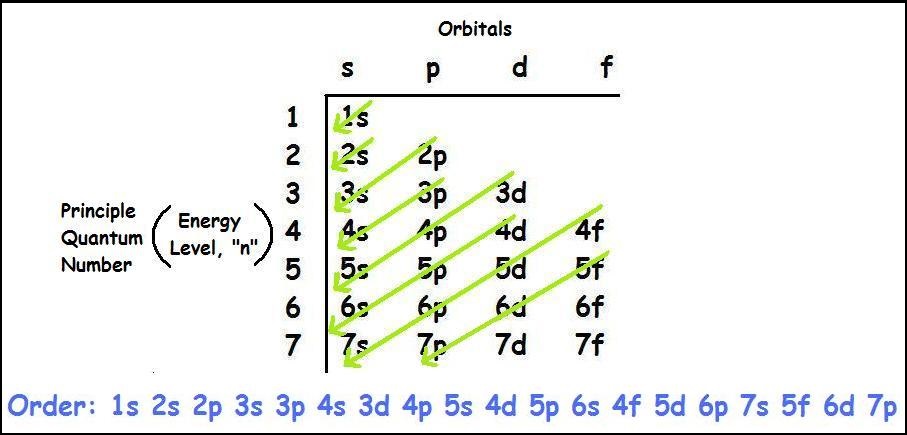
The Aufbau principle is a basic rule that describes the order in which electrons fill the energy levels and sublevels of an atom. These levels are further divided into sublevels, which are named s, p, d, and f, and each sublevel has a certain number of orbitals that can hold electrons. The third energy level can hold up to 18 electrons, and the fourth energy level can hold up to 32 electrons. The first energy level can hold up to 2 electrons, while the second energy level can hold up to 8 electrons. Each energy level has a certain capacity for electrons, and the electrons fill these levels in a specific order. Protons and neutrons are found in the nucleus of an atom, while electrons orbit around the nucleus in energy levels or shells. The Basics of Electron ConfigurationĪtoms are composed of protons, neutrons, and electrons. In this article, we will explore the concept of electron configuration, how it is determined, and its significance in chemistry. It describes the distribution of electrons among the energy levels and orbitals in an atom and helps to explain the chemical and physical properties of elements.

Electron configuration refers to the arrangement of electrons in an atom, molecule, or ion.


 0 kommentar(er)
0 kommentar(er)
